Course Curriculum
| Module 01: Overview to European Regulations on Medical Devices |
|
Overview to European Regulations on Medical Devices |
|
00:43:00 |
| Module 02: Essential Components of EU MDR |
|
Essential Components of EU MDR |
|
00:40:00 |
| Module 03: Reporting Requirements and Identification |
|
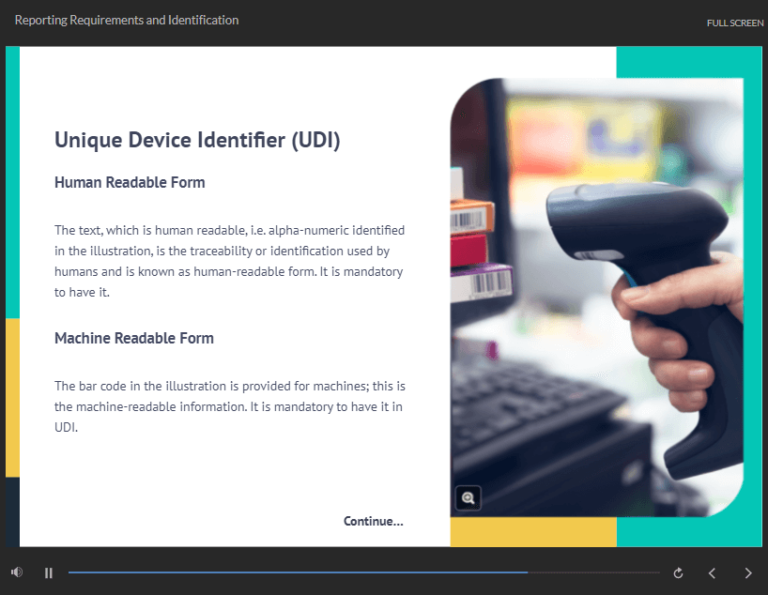
Reporting Requirements and Identification |
|
00:27:00 |
| Module 04: Quality System in Medical Device Regulation |
|
Quality System in Medical Device Regulation |
|
00:36:00 |
| Mock Exam |
|
Mock Exam – Essentials of European Medical Device Regulations |
|
00:20:00 |
| Final Exam |
|
Final Exam – Essentials of European Medical Device Regulations |
|
00:20:00 |
| Certificate and Transcript |
|
Order Your Certificates or Transcripts |
|
00:00:00 |
Essentials of European Medical Device Regulations





 Gift this course
Gift this course